When comparing protein abundance across treatment groups using Western blotting, it is critical to have a method to account for variation due to errors in loading or protein transfer. A common approach is the use of a loading control, a constitutively expressed protein that does not vary among treatment groups, to normalize the relative expression of the protein of interest. Selection of an appropriate loading control is important and can be difficult. Here we present a comprehensive review of loading controls for Western blots, and the survey results from formal publications.
Western blotting is commonly used to investigate the change in abundance of a specific protein under different conditions. The Western blotting process involves multiple steps, including sample preparation, sample loading, electrophoresis, protein transfer to a membrane, antibody incubation, and signal detection. To interpret the results from any Western blot experiment, a loading control is critical.
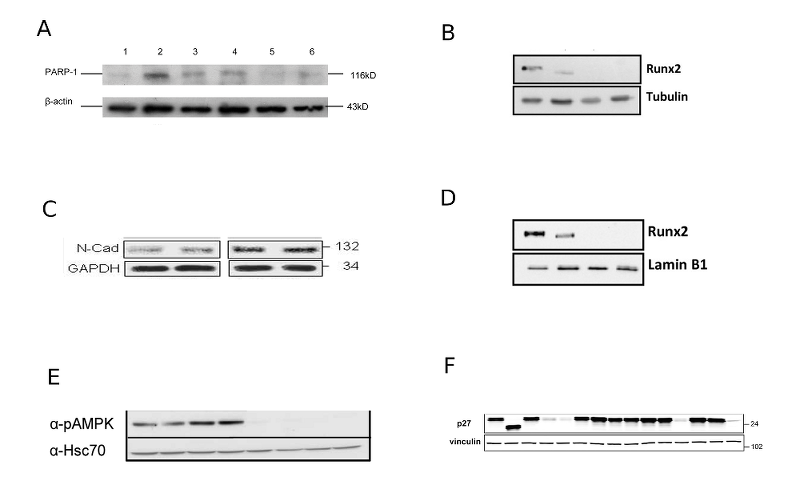
A loading control accounts for potential variation in:
- - the amount of protein sample loaded in each lane,
- - protein transfer efficiencies from the gel to the membrane among different samples/lanes,
- - antibody incubation (for primary or secondary antibody), and signal detection across separate samples/lanes.
The signals from loading controls are typically used to normalize the signals from the proteins of interest. To use a loading control for these purposes detection with control protein antibody and the experimental antibody should be done on the same blot. A variety of different proteins are used as loading controls. For any particular experiment it is important to consider potential loading controls and try to find a protein that is expressed at stable levels among the samples of interest. For this reason housekeeping genes that are constitutively expressed and necessary for basic cell functions, like cytoskeletal proteins, are typically used for loading controls. Looking to the literature can be very useful in considering potential loading controls for your Western blotting experiments.
Labome has surveyed publications with loading controls in Western blots (Table 1). Figure 1 displays the Western blot images compiled from some of those publications. The results indicate that actin (specifically beta actin) is the most commonly used control. Other interesting observations from this survey are discussed in this article later.
| Supplier | Num | Sample Catalog Number with Reference |
|---|---|---|
| actin | ||
| MilliporeSigma | 42 | A3854 A5316 [7] ; A1978 [8, 9] |
| Santa Cruz Biotechnology | 17 | sc-81178 [10] ; sc-69879 / AC-15 [11] |
| Abcam | 4 | ab8226 [12, 13] |
| Cell Signaling | 3 | 4970 [14-16] |
| Novus Biologicals | 1 | NB600-501 [17] |
| MP Biomedicals | 1 | ICN691001 [18] |
| tubulin | ||
| MilliporeSigma | 16 | 05-661 [19, 20], T5326 [21] |
| Santa Cruz Biotechnology | 6 | sc-53029 [22] |
| BioLegend | 2 | MMS-435P [23, 24] |
| Abcam | 1 | ab6160 [25] |
| Cell Signaling Technology | 1 | 3873 [26] |
The proteins serving as loading controls must fit certain criteria. These criteria are:
- the protein levels of loading controls remain constant (relative to the total protein content) under the test conditions (such as across treatments or developmental stages),
- the detection bands of loading controls must not interfere with those of the protein(s) of interest (i.e., they should have substantially different molecular weights),
- the detection limits for the loading controls and the proteins of interest are within dynamic ranges. That is, the protocol and detection method can reveal the changes in the levels of loading controls and the proteins of interest without signal saturation.
The dynamic detection range for a loading control can be determined by running a Western blot specifically for the protein control in serial dilution. The signal intensity must correlate directly with the protein concentration within the range that will be used in the experiment.
Different sample preparations require different loading controls. Table 2 summarizes commonly used loading controls for whole cell/cytoplasmic proteins, mitochondria, nuclear proteins, plant tissues, and serum samples. Each of them is discussed in detail below.
| sample type | protein | MW (kDa) |
|---|---|---|
| whole cell / cytoplasmic proteins | ||
| beta actin | 43 | |
| alpha actin | 43 | |
| GAPDH | 30-40 | |
| beta-tubulin | 55 | |
| alpha-tubulin | 55 | |
| (high molecular weight) | vinculin [27] | 116 |
| mitochondria | ||
| VDCA1/porin | 31 | |
| cytochrome C oxidase | 16 | |
| nuclear proteins | ||
| lamin B1 | 66 | |
| TATA binding protein TBP | 38 | |
| PCNA | 29 | |
| histone H1 | 30 | |
| histone H3 | 18 | |
| plant tissue | ||
| LHCP | 25 | |
| APX3 | 32 | |
| serum | ||
| transferrin | 77 | |
| muscle | ||
| SDHA [28] | 73 | |
| yeast | ||
| phosphoglycerate kinase | 45 | |
Actins are a family of six proteins in three groups in human and other vertebrates: alpha cardiac muscle 1 ( ACTC1), alpha 1 ( ACTA1, skeletal muscle) and 2 ( ACTA2, aortic smooth muscle), beta ( ACTB), gamma 1 ( ACTG1) and 2 ( ACTG2, enteric smooth muscle). Beta and gamma 1 are two non-muscle actin proteins. They function as the main components of microfilaments (Figure 2). Actins are highly-conserved. Beta-actin proteins from species as diverse as human, mouse, and chicken are identical. Human beta actin shares close to 90% identity with fungal homologs. Human beta actin is at least 93% identical with other members of the human actin family. This homology among actins and across species is important for antibody selection and the interpretation of Western blot bands.
Both beta and alpha actins have been used as loading controls in Western blot experiments. Please see the detailed discussion about beta-actin loading control and commonly used clones, and frequently asked questions about beta-actin controls.
Actin proteins tend to be present in cells at very high concentrations, and thus if this control is used it is essential to ensure that you are working within the dynamic detection range so there is no signal saturation and variation in actin protein levels can be detected.
Before selecting an actin protein as your loading control you should ensure that it is an appropriate control with very little to no variation among your treatment groups. Changes in cell growth conditions do disrupt actin protein synthesis. Publications have indicated that beta-actin may not be a reliable loading control in Western blot analysis in most cases [29]. Beta-actin is also present in the nucleus, as a component of chromatin remodeling complexes [30], but it can not be used as a control for nuclear protein samples.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, G3PD, GAPD; Figure 3) catalyzes the reversible oxidative phosphorylation of glyceraldehyde-3-phosphate in the presence of inorganic phosphate and nicotinamide adenine dinucleotide (NAD) in glycolysis. It also has nitrosylase activity and is involved in gene transcription, RNA transport, DNA replication, and apoptosis.
GAPDH is a housekeeping gene and used as controls in both Western blot and qPCR. GAPDH is highly conserved across species. For instance, human GAPDH with 335 amino acids shares about 70% identity with its 422 amino acids homolog from Arabidopsis thaliana. It is present in the cytosol, nucleus, perinuclear regions, and membranes. A comprehensive study indicates that GAPDH mRNA levels differ significantly among tissue types, but remain constant with regards to age and gender [31]. Hypoxia can upregulate GAPDH expression [32, 33], and GAPDH can aggregate [34]. Thus it may be a useful loading control in some Western blot analyses but can not be reliable in other cases, such as for oxygen-related studies. Interestingly, it is the target of dimethyl fumarate, an immunomodulatory drug used to treat psoriasis and multiple sclerosis, which succinates and inactivates the catalytic cysteine of GAPDH [35]. In addition, GAPDH is an RNA-binding protein [36].
There are five types of tubulin: alpha, beta, gamma, delta, and epsilon. Alpha-, beta-, and gamma-tubulins have all been used as loading controls.

Heterodimers of alpha- and beta-tubulins are the building blocks of microtubules (Figure 4). Subtypes of alpha-tubulins include 1a (TUBA1A), 1b (TUBA1B), 1c (TUBA1C), 3c (TUBA3C), 3d (TUBA3D), 3e (TUBA3E), 4a (TUBA4A), and 8 (TUBA8). The subtypes of beta-tubulins are Classes I (TUBB), IIa (TUBB2A), IIb (TUBB2B), III (TUBB3), IVa (TUBB4A), IVb (TUBB4B), V (TUBB6), VI (TUBB1), and VIII (TUBB8).
Gamma-tubulins, gamma 1(TUBG1) and 2 (TUBG2), are involved in the nucleation and polar orientation of microtubules, and are present in centrosomes and spindle pole bodies. Delta (TUBD1) and epsilon (TUBE1) tubulins tend to localize at centrioles and are structural components of mitotic spindles.
Tubulins are conserved across species. For example, human tubulin alpha 1a with 451 amino acids share 74% identity with its yeast homolog Tub3p with 445 amino acids. Subtypes of alpha, beta, or gamma are very similar as well. Human alpha-tubulins share more than 90% identity with one another. Different types of tubulins share significant homology as well. Human alpha-tubulins share 40% identity with human beta-tubulins.
Tubulins are pharmacological targets for several groups of drugs, like anti-cancer drugs Taxol (Figure 4), Tesetaxel, vinblastine, and vincristine, anti-gout agent colchicine, and anti-fungal drug griseofulvin. These drugs affect tubulin expression in vitro and in vivo.
Another cytoskeletal protein, vinculin, has been used as a Western blot loading control as well. It is one of the major components of cell-cell and cell-matrix junctions. It is a large protein; the molecular weight for human vinculin is 117 kDa with 1066 amino acids. It can be used as a loading control for high molecular weight proteins. In muscle tissues, a splice variant with an extra exon with the molecular weight of 150 kDa is also expressed.
Lamin proteins are the structural components of the nuclear lamina (Figure 5), which support nuclear envelope and is involved in the breakdown and re-formation of the nuclear envelope during the cell cycle. In animal cells, three genes encode at least seven lamins. LMNA gene encodes both A- and C-types of lamins through alternative splicing, while LMNB1 and LMNB2 genes encode lamin B1 and B2. B-type lamins are present in every cell. A-type lamins are expressed after gastrulation, and the expression of C-type lamins is tissue-specific.
Post-translational modifications such as farnesylation and phosphorylation occur on lamins, which in turn regulate the assembly of the nuclear lamina and the activity of lamin proteins.
Among the lamins, Lamin B1 is most commonly used as a loading control. Lamin B1 is conserved across species. Human lamin B1 shares 95% identity with rodent homologs, and 36% identity with the fruit fly counterpart. Human lamin B1 is more than 50% identical to other human lamins. However, Lamin B1 protein is not suitable as a loading control for samples without a nuclear envelope.
TATA-box binding protein, TBP, is a general transcription factor that binds specifically to the TATA box DNA sequence before gene transcription by RNA polymerase II. TATA boxes are present in 10-20% of human gene promoters. TBP is highly conserved. Human TBP shares about 90% identity with rodent homologs and over 60-70% identity with fungal homologs. The N-terminus of TBP contains a long string of glutamines (CAG repeats in TBP mRNA molecules), which modulates the DNA binding activity of the C-terminus. The number of N-terminal glutamines in TBP varies in healthy individuals (between 25 to 42 repeats) and expands to 47-63 repeats in certain pathological conditions. This and other post-translational modifications may cause small variation the molecular weight of TBP on Western blots.
Heat shock proteins (HSPs), also called heat shock cognates (HSCs), are a group of proteins which are upregulated under certain stressors, especially under high heat. These proteins have a wide array of functions including providing protection against such stressors and chaperoning proteins around the cell. Some HSPs, such as HSC70 and HSP90 [37], are occasionally used as Western blot loading controls. For example, Galmozzi A et al ensured equal loading of brown adipose tissue with GeneTex anti-HSP90 antibody ( GTX101423) [38]. Wang C et al used HSP90 as a loading control for Hep3B, Huh7, and other human liver cancer cell lines with different treatments [39]. While some HSPs may be constitutively expressed and thus may serve as a reliable loading control in some Western blot analyses, it is critical to keep in mind that treatment conditions may alter expression of certain HSPs.
Proliferating cell nuclear antigen (PCNA) is involved in eukaryotic DNA replication. It is a cofactor of DNA polymerase delta, and is expressed in the nuclei of cells during the DNA synthesis phase (S phase) of the cell cycle. PCNA is highly conserved among chimpanzee, dog, cow, mouse, rat, chicken, zebrafish, fruit fly, mosquito, S. pombe, S. cerevisiae, K. lactis, E. gossypii, M. grisea, N. crassa, A. thaliana, and rice. Human PCNA, with 261 amino acids, shares 97% identity with rodent homologs and 36% identity with yeast homologs. However, because the function of PCNA is in DNA replication it is a poor loading control for non-proliferating cells or cells with anti-proliferation treatment.
Voltage-dependent anion channels (VDCAs), a class of porin family proteins, constitute the major proteins of the outer mitochondrial membrane in eukaryotic cells. There are at least three members (VDAC1, VDAC2, and VDAC3) in vertebrates. VDAC1 is present in both the outer mitochondrial membrane and the plasma membrane, serving different functions in each location. It is expressed in heart, liver, and muscle tissues. Multiple variants of VDAC1 exist due to alternative splicing. The VDAC1 gene is conserved among chimpanzee, dog, cow, mouse, rat, chicken, and zebrafish. Human VDAC1 share 99% identity with the mouse homolog and 85% identity with its zebrafish homolog.
Cytochrome C oxidase, in the inner mitochondrial membrane, is the last enzyme complex of the mitochondrial electron transport chain. The complex consists of 13 subunits encoded by both mitochondrial and nuclear genes.
Subunit IV of cytochrome C oxidase has two isoforms (isoforms 1 and 2). They are encoded by two nuclear genes (COX4I1 and COX4I2). Isoform 1 is ubiquitously expressed, and isoform 2 is highly expressed in lung tissues. Isoform I COX4I1 is frequently used as a loading control. Human COX4I1 shares 80% identity with the mouse homolog, 63% identical to its zebrafish homolog, and 60% identity with human COX4I2.
Transferrin is a blood plasma glycoprotein sometimes used as a Western blot loading control. Its major function is to transport iron from the liver, intestines and reticuloendothelial system to proliferating cells throughout the body. Human transferrin, with 698 amino acids, is 73% identical to its mouse homolog and 43% identical to the zebrafish homolog. When considering Transferrin as a loading control it is important to be aware that its expression is affected in some inherited diseases and by retinoic acid treatment.
APX3, ascorbate peroxidase 3, has been used as a Western blot loading control for plant membrane fractions. It is a microsomal ascorbate peroxidase, which scavenges hydrogen peroxide in plant cells. In Arabidopsis there are three cytosolic (APX1, APX2, APX6), two chloroplastic types (stromal sAPX, thylakoid tAPX), and three microsomal (APX3, APX4, APX5) isoforms.
Ling Q et al used Slp1 and Tic110 as loading controls for western blot in Arabidopsis protoplast lysates [40].
Several authors have proposed to forego the use of a protein as a loading control, and to rely on the dye staining of proteins before (by Coomassie blue [41] ) or after (by Ponceau [42] or, more recently, REVERT [43, 44] ) the transfer step during Western blotting, or use a Stain-Free technology (adding a 58-Da Trihalo compound to the gel) [45]. The results for the protein of interest can then be compared to the total protein rather than a selected loading control that may vary unexpectedly among treatment groups, leading to inaccurate results.
The problem with such alternatives to loading controls is that they may not be able to account for all three considerations of an ideal control: sample loading, protein transfer, and antibody incubation/signal detection. Therefore, a combination of these processes, a protein loading control, Coomassie blue staining of the gel, and Ponceau staining of the transfer membrane, constitutes a well-devised Western blot protocol and thus should be considered. In this way you can confirm your observed results are consistent and thus more robust.
Antibodies from many suppliers have been used to detect different types of loading controls among the publications Labome surveyed.
Santa Cruz anti-beta-actin antibodies served as loading controls in A549 whole cell lysates, HUVEC and dermal fibroblast lysates ( sc-47778) [21, 46] and intestinal epithelial cells from mouse terminal ileum [47].
MilliporeSigma anti-actin antibodies were used for loading controls in Western blots examination [48, 49].
Abcam anti-actin antibodies were used in studies involving K562-TP53 isogenic AML cell lines [13], mouse peritoneal macrophage and human RAW264.7 cells [12].
Thermo Fisher anti-beta-actin antibody was used as a loading control with human carcinoma cell lines to investigate the role of Brachyury in tumor cell epithelial-mesenchymal transition and tumor progression [50].
Chakraborty AA et al used Cell Signaling actin antibody (catalog: 4970) at 1:5000 as a loading control with human mammary epithetelial cells [15]. Zeng Q et al used the same antibody as a loading control for the western blot analysis of N-methyl-D-aspartate receptors in human MDA231 and mouse TS1 breast cancer cells and their corresponding breast-to-brain metastasis derivative cells [14].
In the articles examined by Labome, alpha-, beta-, and gamma-tubulins have been used as loading controls.
Santa Cruz Biotechnology anti-alpha-tubulin antibody was used in an optoSOS study (catalog number: sc-23948) [51] and in transfected U937 cells [52]. Patzke C et al used immunoblotting with anti-tubulin beta III mouse monoclonal TuJ1 antibody from BioLegend / Covance as a loading control for human induced neuron lysates [24].
MilliporeSigma anti-tubulin antibodies were used as loading controls with NIH3T3 cells 05-661 [20], A549 cells (a pd:4409345>T5326) [21], HEK293T [53], and C2C12 cells [15]. Hamilton WB et al detected tubulin in ES cell lysates with Abcam ab6160 to serve as a loading control [25].
Moya IM et al used antibody AM4300 from Thermo Fisher to stain GAPDH in Western blot to ensure equal loading of mouse liver lysates [54]. Laflamme C et al used OriGene antibody ( TA802519) to stain the lysates from HEK-293 cells [44]. Santa Cruz Biotechnology anti-GAPDH antibody was used as loading controls in HepG2 and HeLa cell lysates [16] or lysates of primary myoblasts and fibroblasts ( sc-47724) [55]. Abcam monoclonal anti-GAPDH antibody ( ab8245) was used in Western blotting as a loading control in choroid plexus organoids [56] and 3T3 cell lysate [57]. Li L et al used the GAPDH antibody from Yeasen (
30201ES20) [58]. MilliporeSigma anti-GAPDH antibodies were used as controls in lysates of U2OS cell lines [59], zebrafish ventricles [60], 293T cells expressing pseudotyped viruses ( G8795) [61], human circulating tumor cells ( ABS16 [62] ), ES cells ( G8795) [25], extracted mouse epithelial cells [63] and in the cytoplasmic fractions of mouse livers and HepG2 cells ( MAB374) [49].
GAPDH antibodies from CST clone 14C10 [46, 64, 65], D46CR [66] and D16H11 [67] were used as well. Choi JH et al used D16H11 GAPDH antibodies from CST as a cytosolic marker [67]. Zeng Q et al used the CST GAPDH antibody ( 2118) as a loading control for the western blot analysis of N-methyl-D-aspartate receptors in human and mouse breast cancer cells and their corresponding breast-to-brain metastasis derivative cells [14].
de Morree A et al compared the expression of Pax3 protein in muscle stem cells between wild-type and Pax3-KO mice and among the wild-type mice treated with various antisense vivo-morpholino oligonucleotides in Peggy Sue capillary western using vinculin detected by MilliporeSigma V9131) as a loading control [68]. Lundby A et al detected A549 whole cell lysates with anti-vinculin antibody V9264 from MilliporeSigma [21]. Chakraborty AA et al used vinculin antibody from MilliporeSigma ( V9131) at 1:5000 on C2C12 cell lysates [15]. Vasan N et al measured the sample loading of MCF10A, NIH-3T3 and MCF7 cell lysates with the anti-vinculin antibody (13901) from from Cell Signaling Technology [69].
A variety of other protein targets have been selected to serve as loading controls under a myriad of circumstances. Li Z et al used spectrin, detected with the MilliporeSigma MAB1622 antibody as a control for the lysates from mouse cortices and cultured mouse cortical neurons [70]. Zeitler B et al used calnexin as a loading for lysates of Huntington’s disease fibroblast line GM04723 cells [71]. Engle DD et al normalized Western blot signals from organoid lysates against cofilin, an actin-binding protein which disassembles actin filaments [72]. Tornabene P et al measured protein expression in retinal lysates from mice and pigs with dysferlin detected with clone Ham1/7B6 (MONX10795) from Tebu-bio as controls [17]. Choi JH et al used histone H3 with Cell Signaling Technology antibody ( 9715) as a nuclear fraction marker for Western blot [67]. Saito T et al used lamin B as a nuclear fraction marker for lysates from mouse livers and HepG2 cells [49]. Transferrin was used as a loading control for serum samples [73]. The papers examined by Labome also found lamin A/C [74], histone H3 [67, 75] used as Western blot loading controls.
- Dittmer A, Dittmer J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27:2844-5 pubmed
- Olave I, Reck Peterson S, Crabtree G. Nuclear actin and actin-related proteins in chromatin remodeling. Annu Rev Biochem. 2002;71:755-81 pubmed
- Barber R, Harmer D, Coleman R, Clark B. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389-95 pubmed
- Yamaji R, Fujita K, Takahashi S, Yoneda H, Nagao K, Masuda W, et al. Hypoxia up-regulates glyceraldehyde-3-phosphate dehydrogenase in mouse brain capillary endothelial cells: involvement of Na+/Ca2+ exchanger. Biochim Biophys Acta. 2003;1593:269-76 pubmed
- Zhong H, Simons J. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem Biophys Res Commun. 1999;259:523-6 pubmed
- Minagawa I, Fukuda M, Ishige H, Kohriki H, Shibata M, Park E, et al. Relaxin-like factor (RLF)/insulin-like peptide 3 (INSL3) is secreted from testicular Leydig cells as a monomeric protein comprising three domains B-C-A with full biological activity in boars. Biochem J. 2012;441:265-73 pubmed publisher
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- gene
- human ACTC1
- human ACTG1
- human ACTG2
- human COX4I1
- human LMNB2
- human PCNA
- human TBP
- human TUBA1A
- human TUBA1B
- human TUBA1C
- human TUBA3C
- human TUBA3D
- human TUBA3E
- human TUBA8
- human TUBG2
- human VDAC1
- human actin
- human alpha-tubulin
- human beta-actin
- human delta tubulin
- human epsilon tubulin
- human gamma-tubulin
- human glyceraldehyde 3 phosphate dehydrogenase
- human lamin A/C
- human lamin B
- human smooth muscle actin
- human transferrin
product- Abcam Anti-Tubulin antibody [YL1/2]
- Abcam Anti-GAPDH antibody
- BioLegend Purified anti-Tubulin β 3 (TUBB3)
- Invitrogen GAPDH Monoclonal Antibody (6C5)
- MilliporeSigma Monoclonal Anti-β-Actin antibody produced in mouse
- MilliporeSigma Anti-GAPDH antibody, Mouse monoclonal
- MilliporeSigma Monoclonal Anti-γ-Tubulin antibody produced in mouse
- OriGene GAPDH Mouse Monoclonal Antibody [Clone ID: OTI2D9]
- Santa Cruz Biotechnology alpha Tubulin (B-5-1-2) Antibody
- Santa Cruz Biotechnology GAPDH (0411) Antibody
- Santa Cruz Biotechnology beta-Actin (C4) Antibody
method- Antibody Applications
- Antibody Companies
- Antibody Dilution and Antibody Titer
- Antibody Storage and Antibody Shelf Life
- Antibody Structure and Antibody Fragments
- Beta Actin Antibody
- GFP Antibody
- HA Hemagglutinin Tag Antibody and FAQs
- Mouse Antibody
- Myc Antibody Review
- Phosphotyrosine Antibody
- Rabbit Antibody
- Rat Antibody
- SARS-CoV-2
- Secondary Antibodies Companies
- Secondary Antibodies



